Ensure brand consistency with smart compliance monitoring
In a crowded marketplace, your packaging is often the first — and sometimes only — touchpoint between your brand and the consumer. Every color, font,...
Pharmaceutical labeling is one of the most complex — and risk-sensitive — processes in product packaging. A single artwork or text error can trigger costly recalls, regulatory warnings, or worse: patient harm.
That’s why more life sciences companies are turning to packaging artwork management software like Cway to simplify workflows, strengthen compliance, and accelerate product launches.
Pharma packaging isn’t just about branding — it’s a matter of safety and regulation. Every element on a label, from dosage information to expiry date, must align precisely with approved regulatory texts.
Recent data shows that labeling errors account for nearly 50 % of pharmaceutical recalls. The reason? Manual workflows, scattered feedback loops, and miscommunication between regulatory, quality, and artwork teams.
Pharma organizations need complete control over their artwork lifecycle, with systems that ensure accuracy, traceability, and audit readiness at every step.
Unlike FMCG packaging, pharma labels are governed by strict global regulations:
Good Manufacturing Practice (GMP) and 21 CFR Part 211 govern how labels are created, stored, and approved.
EU Falsified Medicines Directive (FMD) and US DSCSA require serialization and anti-counterfeiting barcodes.
Braille embossing, multi-language layouts, and market-specific regulatory texts make every artwork version unique.
Even a small change — like adding a new marketing authorization holder (MAH) — triggers a full change-control process that must be documented and approved before release.
See how Cway helps teams maintain brand consistency across platforms, automate compliance updates, and manage global packaging artwork efficiently.
Learn How Cway Streamlines Artwork Compliance and Brand Management
Even with experienced teams and strict procedures, pharmaceutical packaging projects can quickly get complicated. Each label passes through many hands — regulatory, quality, marketing, packaging — and often across several countries. Every team adds input, reviews details, or approves changes, and that’s where small gaps can start to appear.
A single label might exist in multiple languages, formats, and pack sizes. When a dosage update or ingredient change comes from regulatory, that same update must be reflected in every version — without missing a single file. If one market uses the wrong text or barcode, it can lead to rework, delays, or even recalls.
Add to that the pressure of deadlines, audits, and market-specific rules, and even a well-organized process can become hard to control. Most issues don’t come from carelessness but from too many files, too many steps, and not enough visibility — which is exactly where a structured system like Cway can help teams keep everything connected and accurate.
Here are some of the most common challenges pharma teams face every day:
Pharma artwork often goes through several updates — text revisions, logo changes, new dosage instructions, or updated regulatory statements. When these versions are shared by email or stored in folders without clear naming rules, it’s easy to lose track of which one is final. A single outdated file sent to print can mean a full product recall.
Many pharmaceutical companies operate across dozens of markets. Each country has its own language, legal text, and approval team. Without a shared approval system, it’s nearly impossible to know who has approved what. Some markets may work on outdated layouts while others are already using new ones — creating serious compliance gaps.
Every label change in pharma — even minor — must go through a formal change control process.
When handled manually, teams end up spending hours filling forms, forwarding PDFs, and checking email threads. This not only slows down projects but also increases the chance of missing required reviews or signatures.
Manual proofreading takes time and focus. Reviewers compare artwork files side by side, trying to catch changes in text, symbols, or barcodes. It’s easy to miss a small font change, a misplaced number, or a wrong logo color — things that can cause regulatory trouble later. Automatic proofreading tools can help, but not every team has access to them or trusts them yet.
When each department — regulatory, quality, marketing, and packaging — uses separate tools, no one has a clear view of progress. Project managers struggle to answer simple questions like “Is this artwork approved?” or “Which version went to print?”. This lack of visibility makes it hard to meet deadlines and maintain consistency across product lines.
Pharma artwork is closely audited by authorities. Every approval, comment, and file change must be traceable. If records are scattered across inboxes or lost in shared drives, it’s almost impossible to prove compliance. Missing audit trails, incorrect serialization data, or mismatched artwork versions can all lead to warnings, fines, or market withdrawals.
Finding the right artwork management system can feel complicated — especially in the pharmaceutical world, where every label has to meet strict regulatory rules and pass through multiple hands before it reaches the shelf.
The goal isn’t just to make design reviews easier; it’s to stay in full control of every version, approval, and change that happens across markets. That’s exactly where a platform like Cway can make a difference.
Pharma packaging isn’t like other industries. Each file carries legally approved text, barcodes, and safety details. Cway is built to give teams a single, reliable place for all artworks — every file, comment, and version stored with full traceability.
It supports:
Role-based access so only authorized users can edit or approve
Automatic logging of who changed what and when
That means you always know which version is approved and can prove it if needed.
Label design in pharma involves more than just a designer and a reviewer — it brings together regulatory, QA, marketing, and packaging. Cway allows all these people to review and comment on the same file, in one secure workspace.
Instead of long email threads and scattered attachments, feedback appears directly on the artwork.
You can see what’s approved, what still needs attention, and who’s responsible — all without losing track of versions or deadlines.
Pharmaceutical artwork management isn’t one-size-fits-all. Some companies manage hundreds of SKUs across multiple regions; others handle updates for just a few key products. Cway’s workflow setup is flexible — it can match your existing approval routes, product categories, or regional setups.
You can create separate workflows for:
New product launches
Regulatory updates
Label text changes
Rebranding or packaging redesigns
This flexibility helps teams stay organized without needing to change how they already work.
Every action in Cway is logged automatically — approvals, uploads, version changes — which creates a full digital audit trail. For pharmaceutical teams, this kind of traceability is essential for meeting inspection requirements and internal quality standards.
While Cway is used across industries, its structure and controls make it a strong fit for teams that need a clear, validated record of their artwork process.
The best pharma artwork system doesn’t have to be overly technical — it just has to make teams confident that what they send to print is correct. Cway helps with exactly that: clear collaboration, transparent approvals, and version control you can trust.
When the process is under control, teams can focus less on tracking files and more on delivering accurate, compliant packaging that gets products safely to market.
Build a Traceable, Compliant Packaging Process — See Cway Live.

In a crowded marketplace, your packaging is often the first — and sometimes only — touchpoint between your brand and the consumer. Every color, font,...

Endless email chains, delayed approvals, and costly reworks — if this sounds familiar, it’s time to consider automated artwork management. This...
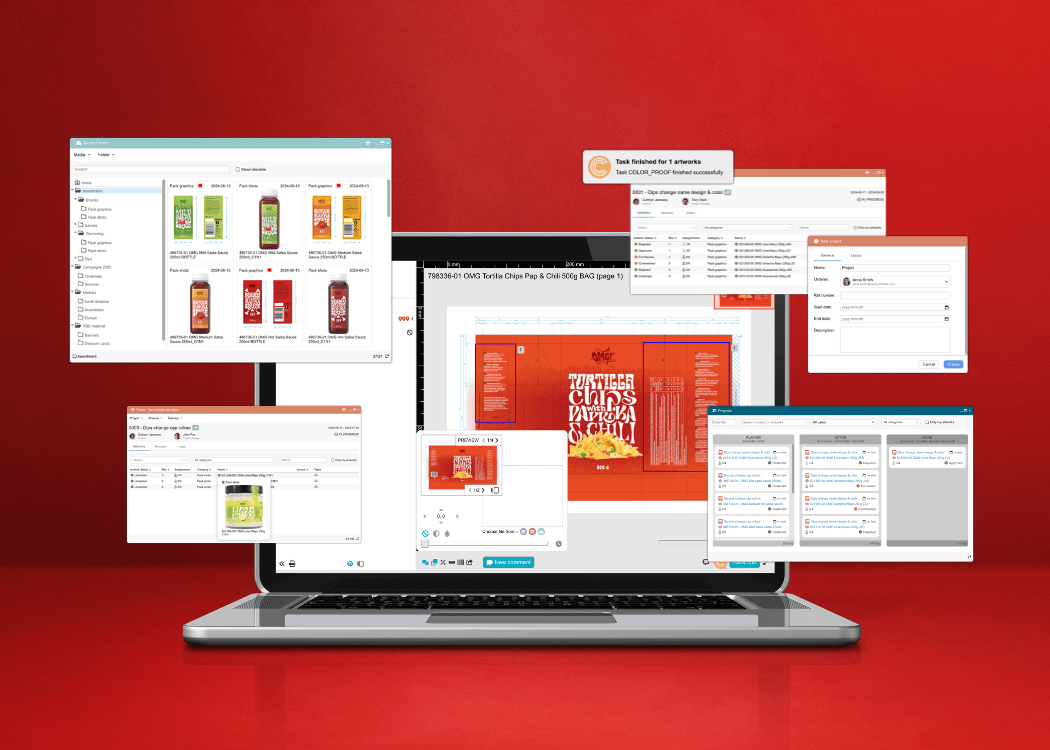
Streamline design approvals, enhance feedback loops, and get to market faster with Cway®—your all-in-one platform for artwork approval process.