Less email, more feedback: the power of proofing and approval software
In today’s fast-paced creative and marketing environments, time is more than money—it’s competitive edge. But too often, projects stall in the final,...

There’s a moment every packaging team knows.
You’re reviewing the artwork…
You spot the requested change…
And your stomach drops just a little.
Because in pharma, a misplaced digit in dosing, a swapped barcode, or a mistranslated warning isn’t “a small mistake.”
It’s a recall. A risk to patient safety. A compliance breach.
High-risk approvals aren’t just a step in the process — they’re the guardrail that keeps everything upright.
And the expectations from regulators? They’re only getting tighter.
Here’s what the top-performing pharma packaging teams are doing today… and where solutions like Cway quietly become indispensable.
Pharma teams don’t rely on gut instinct to classify a change as high-risk.
They rely on regulatory definition.
A few examples:
Dosing, contraindications, warnings → tied to EMA QRD templates + FDA labeling requirements
Serialization or barcodes → governed by EU Falsified Medicines Directive (2011/62/EU) & US DSCSA serialization rules
Braille → required under EU Directive 2004/27/EC, with exact formatting rules
Tamper-evidence updates → linked to EN 16679 standards
If the change affects patient safety, pack integrity, identification, or legal compliance → It’s high-risk. Full stop.
This classification triggers the heavier workflow — and rightly so.
Pharma approvals must satisfy multiple layers of regulatory expectation, including:
GMP (Good Manufacturing Practice)
GDP (Good Distribution Practice)
ICH Q8–Q10 guidelines (quality, risk management, pharmaceutical development)
EMA & FDA labeling rules
Market-specific health authority requirements
Which means approvals aren’t just signatures — they’re regulated handoffs.
Who must be involved?
Regulatory Affairs (ensuring labeling matches approved SmPC/PI)
Quality Assurance (GMP alignment + release control)
Pharmacovigilance (safety statements, adverse event wording)
Medical Affairs (clinical accuracy)
Local Affiliates (market language + legal expectations)
Packaging / Artwork Specialists (technical accuracy + compliance with print specs)
This isn’t workflow.
It’s choreography — and every misstep matters.
If it isn’t captured, timestamped, locked, and audit-ready… it didn’t happen.
To comply with FDA 21 CFR Part 11 and EU Annex 11 requirements for electronic records, packaging teams need:
Secure, permission-controlled access
Traceable audit trails
System-logged approvals
Controlled version histories
Proof that data hasn’t been altered
Manual systems fail here.
Email chains fail spectacularly.
This is where digital artwork systems must meet regulatory-grade integrity.
High-risk artwork reviews aren’t “visual checks.”
They follow structured, validated methods.
Teams rely on tools that support:
ISO 15416 / ISO 15426 barcode verification
Text comparison (OCR) for SmPC-to-artwork accuracy
Braille validation per Marburg Medium and EU packaging rules
Color accuracy within tolerance (ISO 12647)
Serialization checks to meet EU FMD & DSCSA mandates
Human error isn’t the failure — lack of a safety net is.
Once the file is approved, its lifecycle must stay compliant with:
GMP Annex 1, 15 & 21
Packaging and labeling control rules
Batch documentation requirements
Which means:
Only QA/RA can release the final artwork
The version sent to print must be locked
Suppliers must receive the correct, approved file
Every market, every batch must be traceable
And if something goes wrong?
CAPA (Corrective and Preventive Action) kicks in — with artwork teams often at the center of investigation.
Pharma teams don’t need more tools.
They need one place where high-risk approvals actually work the way they need them to.
Cway gives packaging and regulatory teams:
Built around your SOPs — not generic templates.
Every comment, version, timestamp, and decision stored automatically.
Text compare, visual compare, barcode checks, and more — inside the workflow.
Locked master files, released versions, and a traceable history from creation to print.
Everyone sees the same file.
Everyone follows the same process.
Everyone stays aligned — even across multiple regulatory jurisdictions.
Cway isn’t just workflow software.
It’s the operational backbone that helps pharma teams reduce risk, accelerate approvals, and stay compliant without compromising speed.
Cway gives you a single source of truth, automated approvals, and compliant traceability — so your team can focus on patient safety, not process firefighting.
If you’re rethinking how high-risk approvals should work:
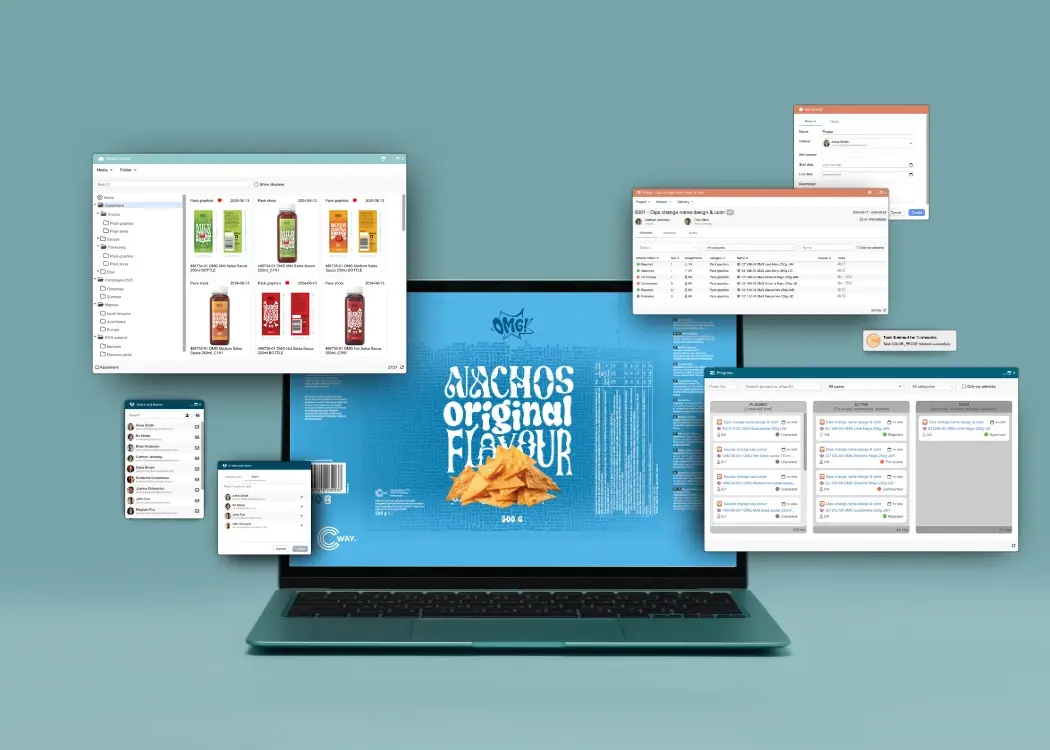
In today’s fast-paced creative and marketing environments, time is more than money—it’s competitive edge. But too often, projects stall in the final,...
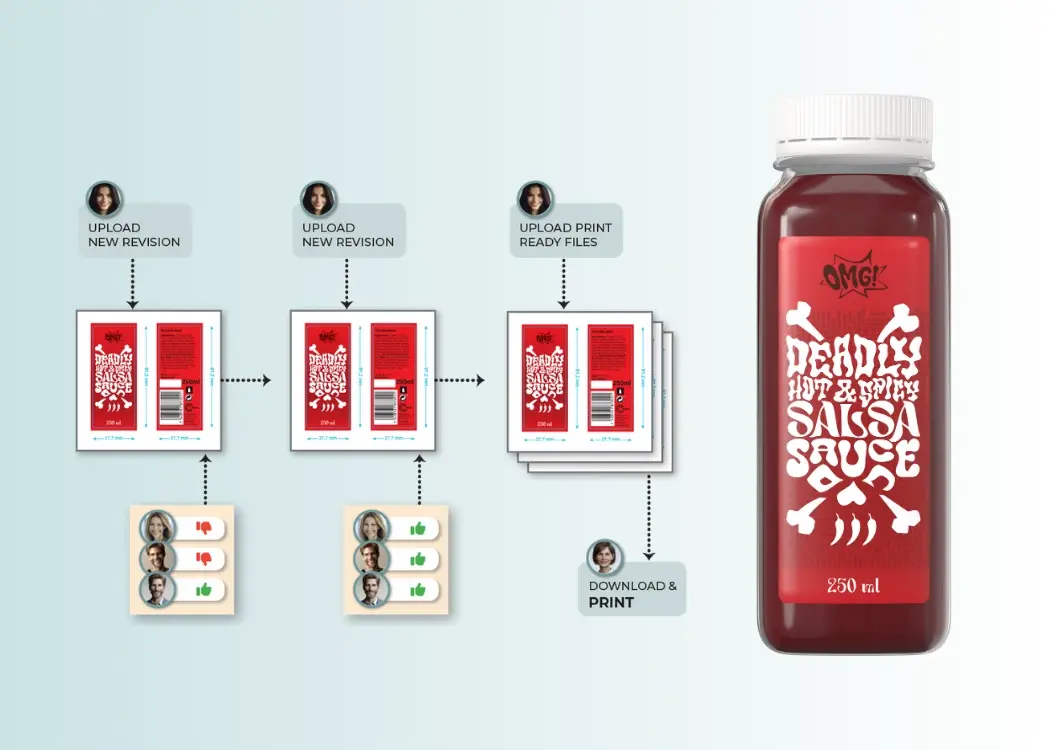
Picture this: a brand is preparing to launch a new product. Designs are flying across email threads, approvals are stuck in endless loops, and the...

Today’s brands win on speed, accuracy, and consistency—and product content sits at the heart of all three. But siloed tools and disconnected...