Sustainable packaging trends 2026 for smarter artwork management
As sustainability standards evolve, packaging teams face growing pressure to adapt quickly — from new materials to changing regulations and label...

If you work in pharmaceutical packaging, you already know the pressure: multiple SKUs, constant artwork revisions, tight launch schedules, and ever-changing regulatory requirements. A single misprint on a label can lead to costly recalls, compliance issues, or even put patient safety at risk.
That’s why artwork management—the process of creating, reviewing, approving, and distributing packaging artwork—has become a strategic function, not just an administrative task.
In this post, we’ll unpack what pharmaceutical artwork management is, why it matters so much, and how modern digital systems can transform your workflow.
At its core, artwork management is all about coordination. Every box, blister pack, label, or leaflet in a pharmaceutical product line must pass through several hands before it reaches production—marketing, regulatory, quality assurance, and manufacturing, to name a few.
Each stakeholder adds input, and every edit must be version-controlled, traceable, and approved. The process has to be watertight.
In pharmaceuticals, this becomes especially complex because every artwork element—from dosage instructions to barcodes—has regulatory implications. A single inconsistency can halt an entire product launch.
That’s where a pharmaceutical artwork management system comes in: a central platform designed to streamline collaboration, maintain compliance, and keep projects moving without errors or delays.
Imagine launching a new product only to discover a packaging error after it hits the shelves. Not only would that trigger an expensive recall, but it could also damage your brand’s credibility and delay future approvals.
Common pain points pharmaceutical artwork teams face include:
Disjointed collaboration — Multiple departments and vendors working across email threads and PDFs.
Version confusion — No clear record of which file is the latest approved artwork.
Regulatory delays — Missing or inconsistent labeling information causing rejections.
Slow approvals — Manual sign-offs and unclear responsibilities stretching timelines.
The result? Delays, compliance risks, and frustrated teams.
An effective artwork management solution tackles all of these by bringing structure and visibility to every step.
Achieve 60% faster approvals, zero errors, and seamless collaboration with Cway.
Today’s packaging artwork management software replaces manual, email-driven processes with centralized, automated workflows.
A modern artwork management system for pharmaceuticals typically includes:
All files live in one secure place—no more version chaos. Teams always know they’re working from the latest approved design.
Smart routing ensures artwork moves through review and approval stages seamlessly. You can track every task’s status, responsible owner, and deadline.
Every change is logged automatically, which means full traceability—a must for regulatory audits.
Built-in validation tools and approval checklists help ensure that all artwork complies with FDA, EMA, and MHRA requirements.
Internal teams and external partners (design agencies, printers, vendors) can collaborate in real time, cutting approval cycles dramatically.
Advanced systems connect seamlessly with your existing enterprise tools, ensuring that approved artwork flows directly into packaging and production.
|
Benefit |
Description |
|---|---|
|
Faster Time-to-Market |
Automated reviews and approvals mean shorter cycles. No more waiting for email replies or chasing signatures. |
|
Fewer Errors, Fewer Recalls |
Version control and structured workflows drastically reduce human error. Every label is correct the first time. |
|
Improved Compliance |
Audit trails and built-in validation make inspection prep easy and transparent. Regulatory teams save hours weekly. |
|
Enhanced Visibility |
Dashboards and reporting tools provide clear insight into progress, bottlenecks, and upcoming deadlines. |
|
Collaboration Without Chaos |
With marketing, regulatory, and packaging teams working in one system, communication improves and accountability increases. |
At Cway, we’ve seen firsthand how packaging artwork bottlenecks can derail pharmaceutical launches. That’s why our approach focuses on streamlining collaboration and ensuring compliance through intelligent automation.
Our solutions are built around three principles:
Clarity – Everyone works from a single source of truth.
Control – Every version, comment, and approval is traceable.
Compliance – Workflows are designed with pharma regulations in mind.
The result? Faster, more reliable artwork delivery—without the stress.
Whether you manage five SKUs or five thousand, our platform scales with your needs, empowering your teams to focus on what truly matters: delivering safe, compliant products on time.

As sustainability standards evolve, packaging teams face growing pressure to adapt quickly — from new materials to changing regulations and label...
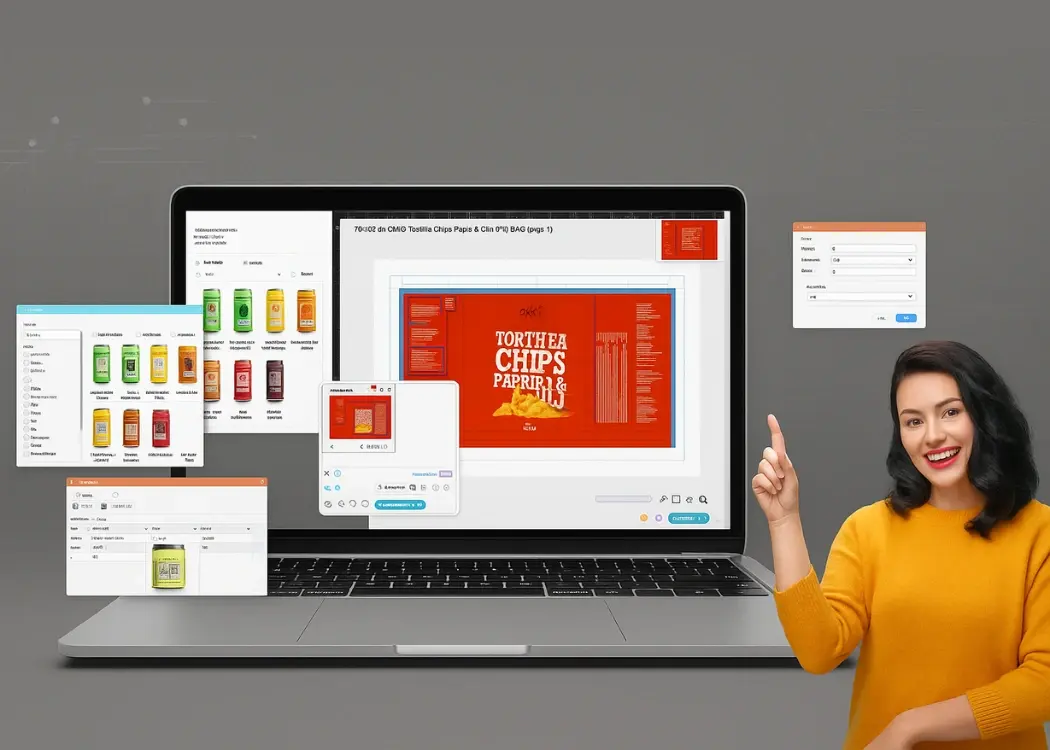
When it comes to packaging, getting every detail right — from regulatory text to design alignment — is crucial. Yet, many teams still manage...
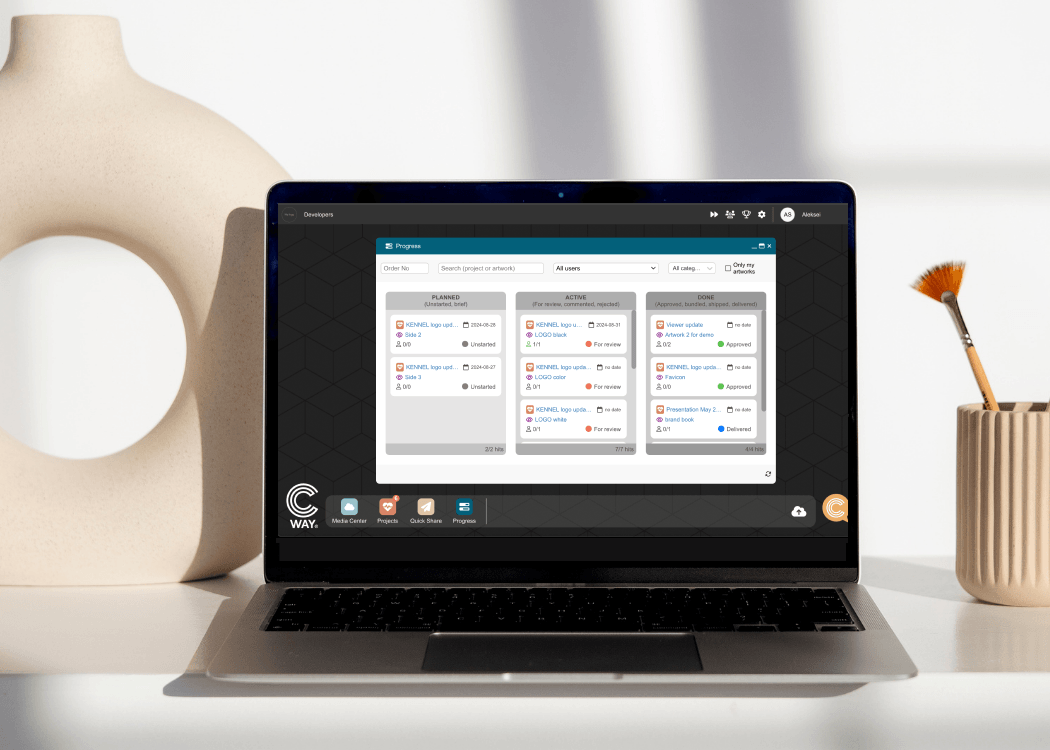
We're thrilled to announce the latest innovation in our suite of tools designed to streamline your artwork management process—Progress, a powerful...